Impact on humans and DNA damage
Humans have limited experience from deep space missions. The International Space Station (ISS) is still fairly well protected by the magnetosphere and only few human beings have ventured beyond this protection zone, and for an extremely short amount of time (compared to the multi-year timeframe for a mission to Mars). Exposure to space radiation increases the risks of astronauts developing cancer, experiencing central nervous system decrements, exhibiting degenerative tissue effects or developing acute radiation syndrome [1,2]. In particular, biological effects of radiation are generally divided into: a) stochastic effects which can significantly increase the likelihood of an astronaut developing late pathologies (e.g., cancer); b) tissue reactions (formerly named deterministic effects), which can cause short-term effects such as cataracts and acute radiation sickness, which will ‘‘definitely’’ occur beyond a threshold dose. Passive shieldings are an effective countermeasure against SEPs, but remain of little efficiency in protecting from fast moving, highly-charged GCRs nuclei and the eventual ramarkable amount of neutrons generated inside a spacecraft/habitat. Astronauts travelling on a protracted voyage to Mars may be exposed to transient intense solar events generating SEPs, overlaid on a more predictable flux of GCRs, not to mention the risks in the case of astronauts on Extra-Vehicular Activity (EVA) missions. At present, active and passive dosimeters are used to determine the radiation dose on the ISS and the depth that such dose reaches in human by means of a human phantom torso (the MATROSHKA experiment [3]).
Damage in biological matter is induced either by direct hit of the impacting particle onto the biological molecules or, and actually mostly, by indirect effects caused by the secondary species generated in the surrounding water medium, such species also attacking the biological molecule. Low energy electrons and several species from water radiolysis cause damage to the DNA molecules and such damages can either be repaired, cause viable mutations (stochastic effects) or induce acute/lethal effects for high doses.
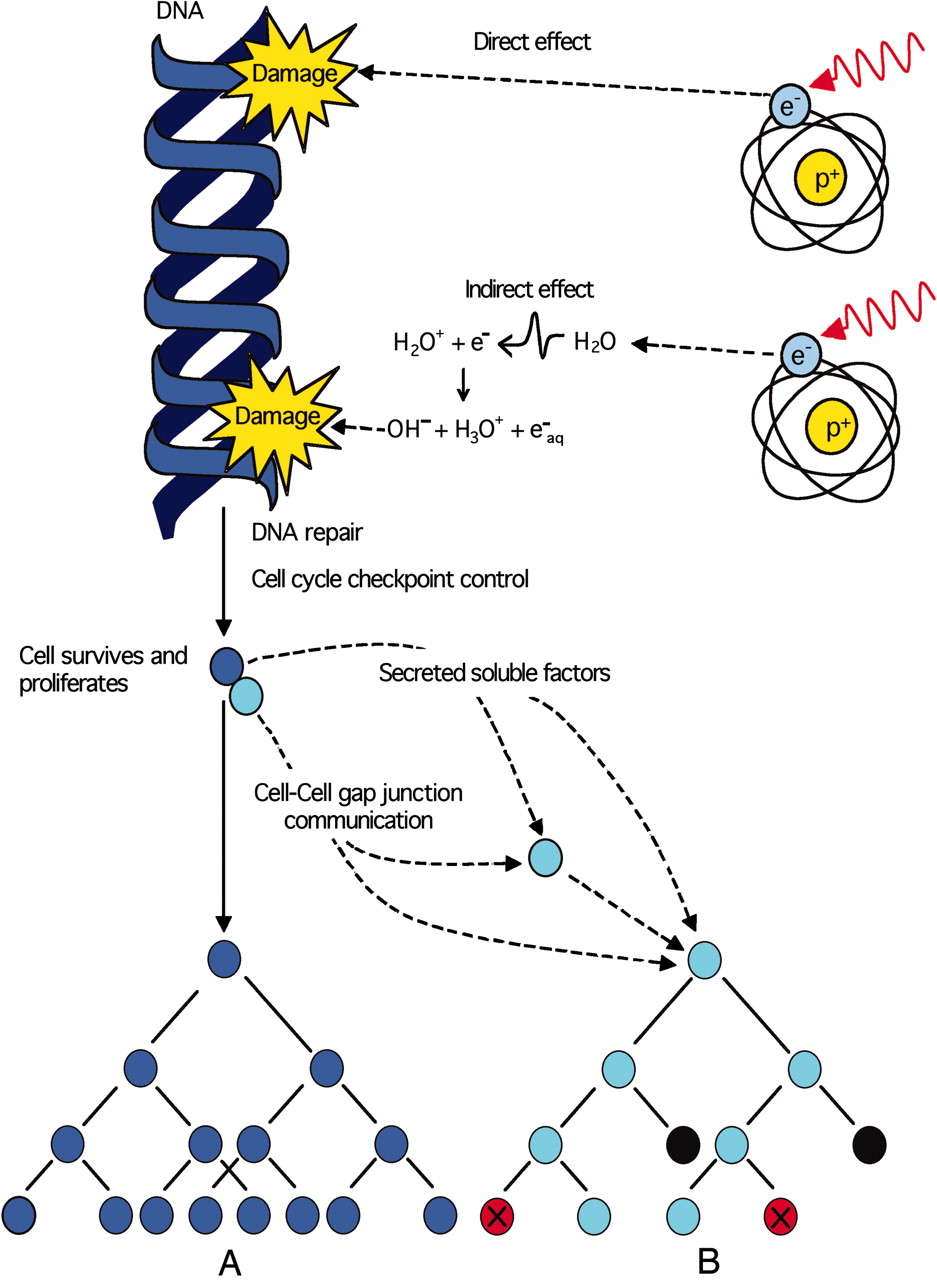
Then, the damage effects occur at different levels: i) at the molecular level, e.g. by bond breakings and changes in the diffusion processes; ii) at the cellular level, e.g. by modifying repair processes or by changing the metabolic/physiological state and/or, iii) at the tissue and organ level, e.g. by modifying self-assembly intercellular communication, cell migration, pattern formation, or differentiation. A significant improvement of theoretical models has to be made in order to understand the damage from the very beginning, i.e. from the very molecular level. Ionizing radiation can damage DNA in a cell either directly or it can excite molecules in the cellular surroundings (water and other molecules of the cells) by creating secondary by- products and excited species that can again act as projectiles and damage DNA (see Figure on the right: Copyright © 2005, The National Academy of Sciences). The damage from direct ionization results from formation of DNA-cation radicals (holes) and DNA-anion radicals (excess electrons) within DNA. The secondary species are low-energy electrons (LEE) or the OH* radicals generated by the radiolysis of water. Only about one third of the cellular damage is produced by direct interaction of the ionizing radiation with DNA, while the rest is actually due to such secondary species [4]. Two principal types of DNA lesions due to ionizing radiation can be mentioned: single strand bond break (SSB), where only one of the two strands of a double helix has a defect, the other strand can be used as a template to guide the correction of the damaged strand; and the double strand break (DSB), in which both strands in the double helix are broken. The mechanisms of damage are extremely complex and the degree of damage is also dictacted by the clustering of lessions in a reduced molecular location and combination with other lession types as those produced in nucleobases [5].
The regime in which the impact particle is ionizing the atoms in the target and generating the secondary species can be, to a certain extent, described quite well in terms of simple sparse collisions nearly independent on each other, with individual water molecules, which is the basic assumption of Monte Carlo simulations in radiation transport tools. The information on scattering cross sections from relatively simple atomic theories is then used to study radiation tracks via such tools [6,7], like Geant4-DNA. Actually, it was previously thought that this ionization regime was the only one relevant for DNA damage, according to the common assumption that maximum energy deposition translates into maximum damage. However, recent studies have shown that there is still a huge amount of damage even when the impact particle is slowed down to an energy such that it cannot ionize the electrons in the target anymore [8]. Within this regime, some approximations on which Monte Carlo tools are based become questionable, as also the use of atomic (gas phase) calculated or measured scattering cross sections.
Geant4-DNA is now the state of the art approach to calculate the (first-stage) effects in biological damage. Nevertheless, some limitations of Geant4-DNA could be overcome nowadays by the first-principles community. Among others, some important limitations are: the cross sections for electronic excitations are calculated in the first Born approximation using optical-data models of ε(E,q) based on a Drude representation of ε(E,q); use of some (ad-hoc) truncation algorithms and re-distribution of the imaginary part of the dielectric function (or oscillator strength) to the different ionization shells and excitation levels in an f-sum-rule conserving manner without affecting the original fit to the experimental data); mostly consideration of the sole water.
[1] J.C. Chancellor, G. B. I. Scott and J.P. Sutton, Life 4, 491-510 (2014) and references therein.
[2] F.A. Cucinotta, M.H. Kim, V. Willingham, K.A. George, Radiat. Res. 170, 127 (2008).
[3] J. Labrenz, S. Burmeister, T. Berger, B. Heber, G. Reitz, J. Space Weather Space Clim. 5, A38 (2015).
[4] B. D. Michael, Science 287, 1603 (2000).
[5] Z. Nikitaki, Ch. E. Hellweg, A.G. Georgakilas and J.-L. Ravanat, Frontiers in chemistry 3, 35 (2015).
[6] M.P. Gaigeot, R.Vuilleumier, C. Stia, M.E.Galassi, R.Rivarola, B.Gervais, M.F.Politis, J. Phys. B: At. Mol. Opt. Phys. 40, 1 (2007).
[7] V. Cobut, Y.Frongillo, J.Patau, T.Goulet, M.Fraser, J.Jay-Gerin, Radiat. Phys. Chem. 51, 85 (1998).
[8] E. Artacho and J.J. Kohanoff, PLOS-ONE (2017).
Contact: BIRA-IASB, Av. Circulaire 3, Brussels, Belgium.
